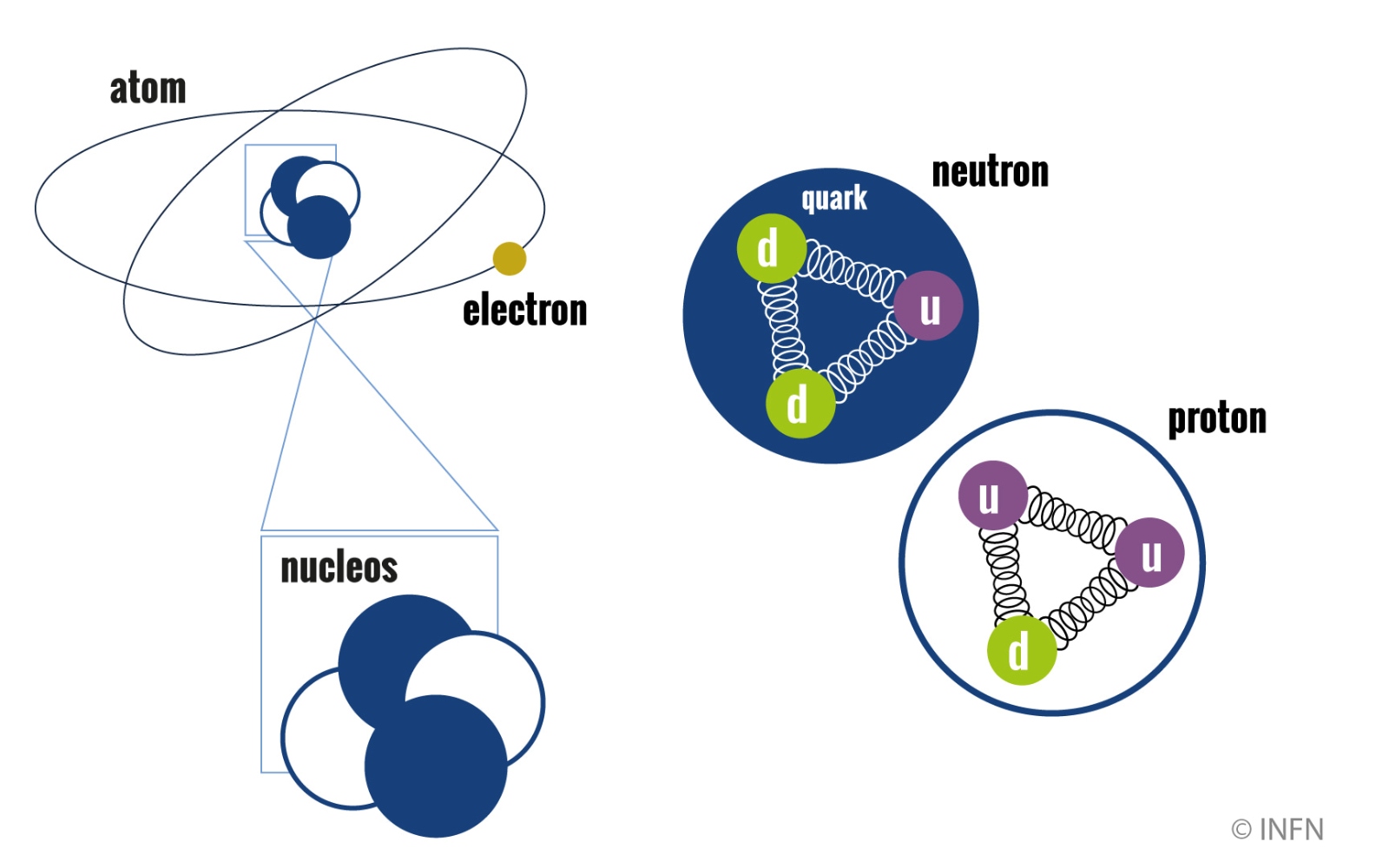
The theoretical and experimental understanding of the atomic and nuclear structure had along journey that involved great scientists. These included Ernest Rutherford, whose experiments at the start of the 1900s were decisive for guessing at the existence of nuclei at the centre of atoms; Niels Bohr, whose atomic model introduced the concept of the quantization of energy and the existence of discrete orbits for electrons around the nucleus; Lise Meitner, with decisive studies on the manipulation of atomic nuclei and nuclear fission, and many others (including, of course, Enrico Fermi and the Via Panisperna Boys).
Increasingly detailed knowledge of the atomic and nuclear structure led to numerous technological applications, including revolutionary ones for modern society (from nuclear medicine to technologies based on lasers, to name just a few). However, in contrast to what one might think, there is still a lot to understand about the behaviour of atoms and nuclei, which are still studied both at a fundamental level and for new potential applications. INFN too has always been at the forefront in this research, with important theoretical and experimental contributions.